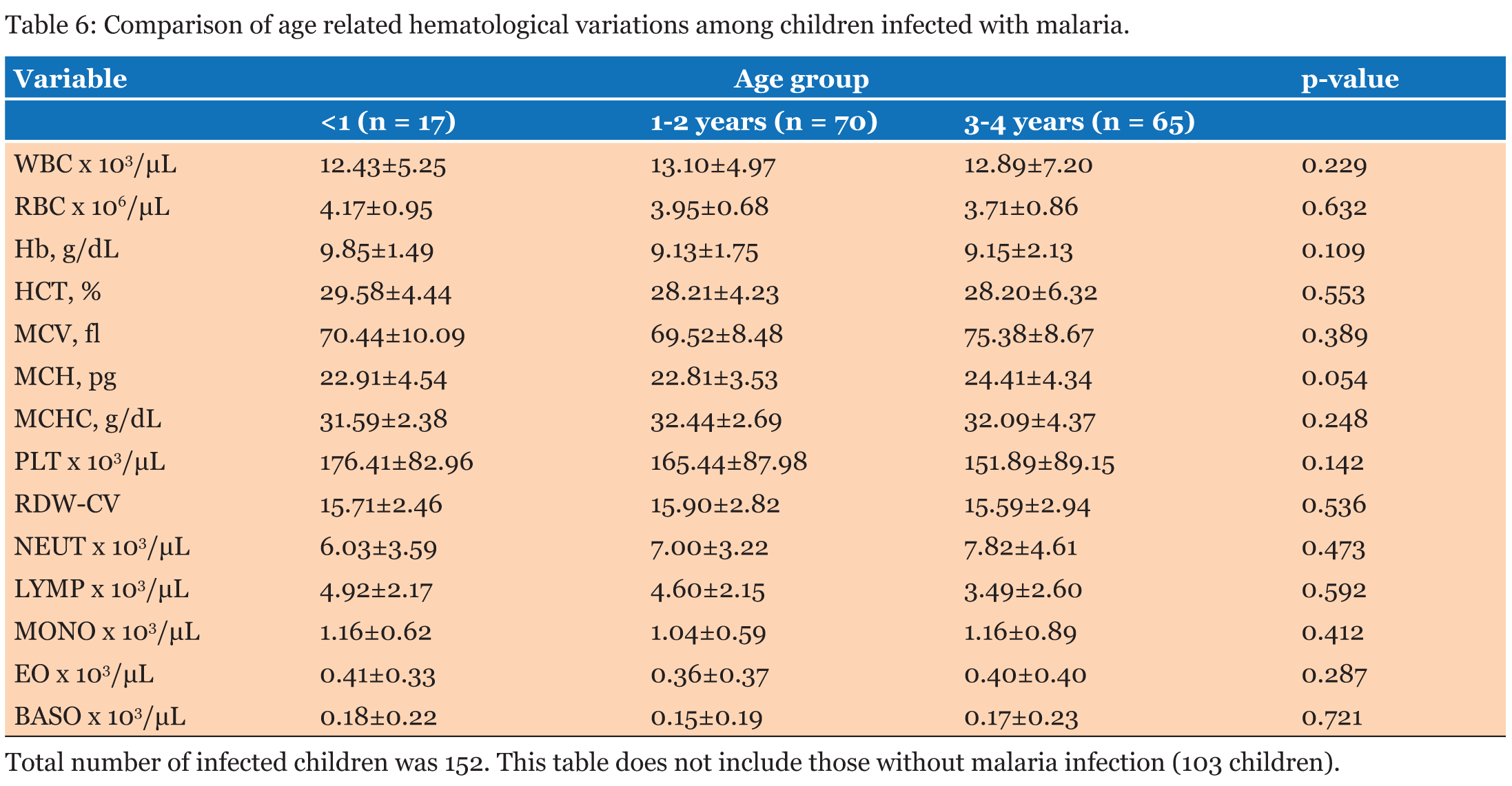|
|
Original Article
| ||||||
| Hematological profile of children under five years with malaria at the Ho Municipality of Ghana | ||||||
| Ahmed Tijani Bawah1, Kofi Theodore Nyakpo2, Francis Abeku Ussher3, Huseini Alidu1, Jerry Jones Dzogbo2, Sampson Agbemenya2, David Annor Kwasie4, Mohammed Mustapha Seini5 | ||||||
|
1Lecturer, Department of Medical Laboratory Science, School of Allied Health Sciences, University of Health and Allied Health Sciences, Ho, Ghana 2Medical Laboratory Scientist, South Tongu District Hospital, Sogakope, Ghana 3Lecturer, Department of Medical Laboratory Sciences, Faculty of Health and Allied Science, Koforidua Technical University, Koforidua, Ghana 4Medical Laboratory Scientist, Volta Regional Hospital Ho, Ghana 5Medical Laboratory Scientist, Greater Regional Hospital, Accra | ||||||
| ||||||
|
[HTML Abstract]
[PDF Full Text]
[Print This Article] [Similar article in PubMed] [Similar article in Google Scholar] |
| How to cite this article |
| Bawah AT, Nyakpo KT, Ussher FA, Alidu H, Dzogbo JJ, Agbemenya S, Kwasie DA, Seini MM. Hematological profile of children under five years with malaria at the Ho Municipality of Ghana. Edorium J Pediatr 2018;2:100004P05AB2018. |
|
ABSTRACT
| ||||||
|
Aims: A lot of efforts have been made to minimize malaria transmission in the world, however, this infection still remains high among humans. Plasmodium infection among children under five is characterized by marked changes in hematopoietic cells as a result of the ability of the parasite to attack and destroy erythrocytes. This study was aimed at elucidating the changes in hematological profile caused by plasmodium parasites among infected children under five years. Methods: A four month, cross sectional hospital-based study was conducted involving 255 children under five years infected with or without malaria parasite. Hematological profiles of the participants were assayed and comparison made with normal reference values. A total of 152 children were infected with malaria parasites with the remaining 103 children unaffected. About 2.5 milliliters of venous blood sample was collected from each participant into K3EDTA tubes. Full blood count and parasite species identification and quantification were done. Results: The hemoglobin concentration and the platelet count were significantly lower among children with malaria infection than those without the parasites and the monocyte count was also significantly higher among children with malaria parasitemia than the control group. The red blood cells (RBC) numbers and the hemoglobin (Hb) level decreased significantly as severity of malaria increased while the monocyte count increased significantly as severity of malaria increased. Conclusion: There are significant changes in the hematological profile of children under five infected with malaria parasites and these changes become profound as the severity of the infection increases Keywords: Anemia, Malaria, Monocytosis parasite density, Thrombcytopenia | ||||||
|
INTRODUCTION
| ||||||
|
Malaria infection is caused by the protozoa plasmodium species; of the genus: Plasmodium; P. falciparum, P. ovale, P. vivax, P. malariae and P. knowlesi, with P. falciparum being the most infectious [1]. It’s transmission from one person to another is carried out through the bite of an infected female anopheles’ mosquito, which acts as the vector. About half of the world’s population resides in malaria endemic areas [2]. Estimates from the world health organization revealed that about 3.2 billion people remain at risk of malaria with the year 2015 alone being responsible for about 214 million new cases and 43800 deaths [3] . Approximately 80% of malaria deaths occur in only 15 countries most of which are in Africa including Ghana [3] . Malaria is a major public health issue in Africa [4]. It is hyper-endemic in Ghana with plasmodium falciparum being responsible for about 90–98% of morbidity and mortality linked to the infection [5]. Undeniably, in Ghana, malaria is the commonest cause of patient admission and death among children. Hematological changes that characterize malaria parasitemia are probably due to the biochemical changes that ensue during the asexual life cycle stage of the plasmodium parasite [1]. The pathological effect of plasmodium infection in children is dependent on many factors including; parasite infectiveness, host susceptibility as well as geographical factors. During the raining season most children are infected with the malaria parasite, which destroys erythrocytes causing significant changes in hematological parameters. Various studies have reported that malaria episode causes many pathophysiological disturbances in its host hematological system including alterations in erythrocytes, leucocytes and thrombocytes subpopulations in the peripheral blood [6]. Hematological alterations may be inducted by several other factors including time after infection, intensity and pattern of transmission of the parasite in the area as well as the strength of host immunity [7]. Several clinical studies have also examined the changes in the peripheral blood counts in uncomplicated malaria infection; few however, have examined and compared these hematological alterations in children under five [8]. Changes in hematological parameters may be influenced by any pathological condition including malaria, which affects the formation, development and differentiation of all types of blood cells [9]. Malaria infection in human is usually accompanied by decrease in hemoglobin concentration leading to severe anemia with a substantial increase in mortality rate [10]. Other factors may also be responsible for this fatality in addition to direct destruction of parasitized red blood cells when merozoites are released [10]. Two national representative survey documents on anemia prevalence states that 84% of children under five, 71% of children of school going age, 65% of women who are pregnant and 59% of breast feeding women were anemic [10]. Anemia is a significant cause of mortality in malaria infection. This may be due to difficulty in diagnosis especially where parasitemia and the clinical picture may be confused with other causes of anemia [10]. The accepted worldwide “gold standard” used for the routine diagnosis of malaria involves microscopic examination of thick and thin blood smears which have been stained with Giemsa for parasite detection and species identification respectively [11]. The major problem with microscopy is that it requires highly trained staff, good quality equipment and reagents. The rest are, regular supply of clean water, electricity, and effective quality management system. Furthermore, there is lack of qualified personnel to carry out microscopy in order to promptly and effectively diagnose malaria in some malaria endemic communities [12]. This has resulted in many efforts being made to replace the use of microscopy to diagnose malaria with other methods such as the rapid diagnostic tests (RDTs) and automated machines including the possibility of using changes in hematological parameters to support the presumptive malaria diagnosis [13]. Alterations in the hematological parameters have also shown the capacity to provide additional tool to increase the suspicion of malaria and thereby facilitate a more vigorous search for the parasites microscopically [14]. To this end, this study was aimed at evaluating the changes in hematological profile of children infected with malaria parasites and elucidates the usefulness of such changes in the diagnosis and management of the disease. | ||||||
|
MATERIALS AND METHODS
| ||||||
|
Study site and design
Sampling
Slide Preparation and Examination
| ||||||
RESULTS | ||||||
|
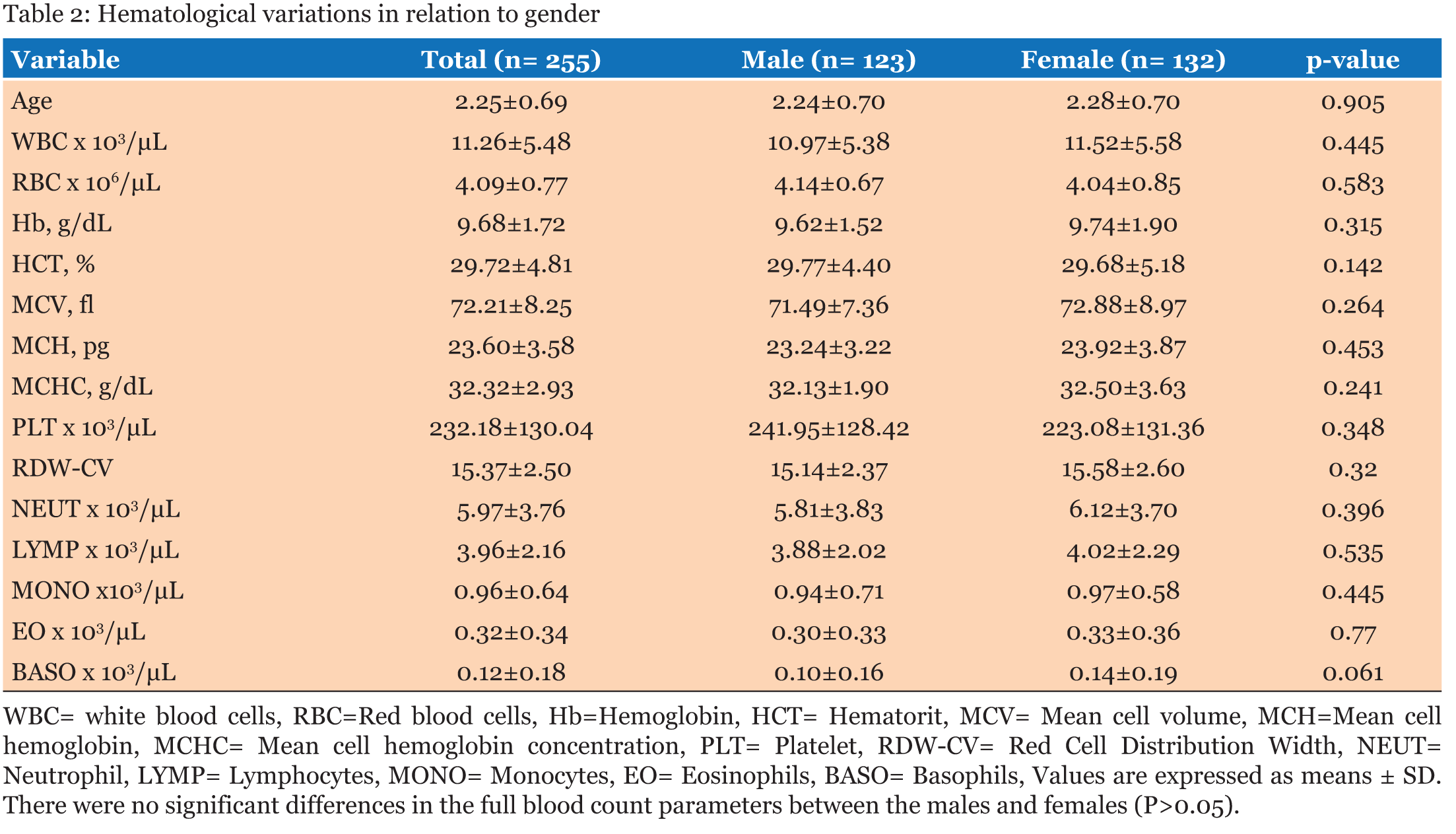
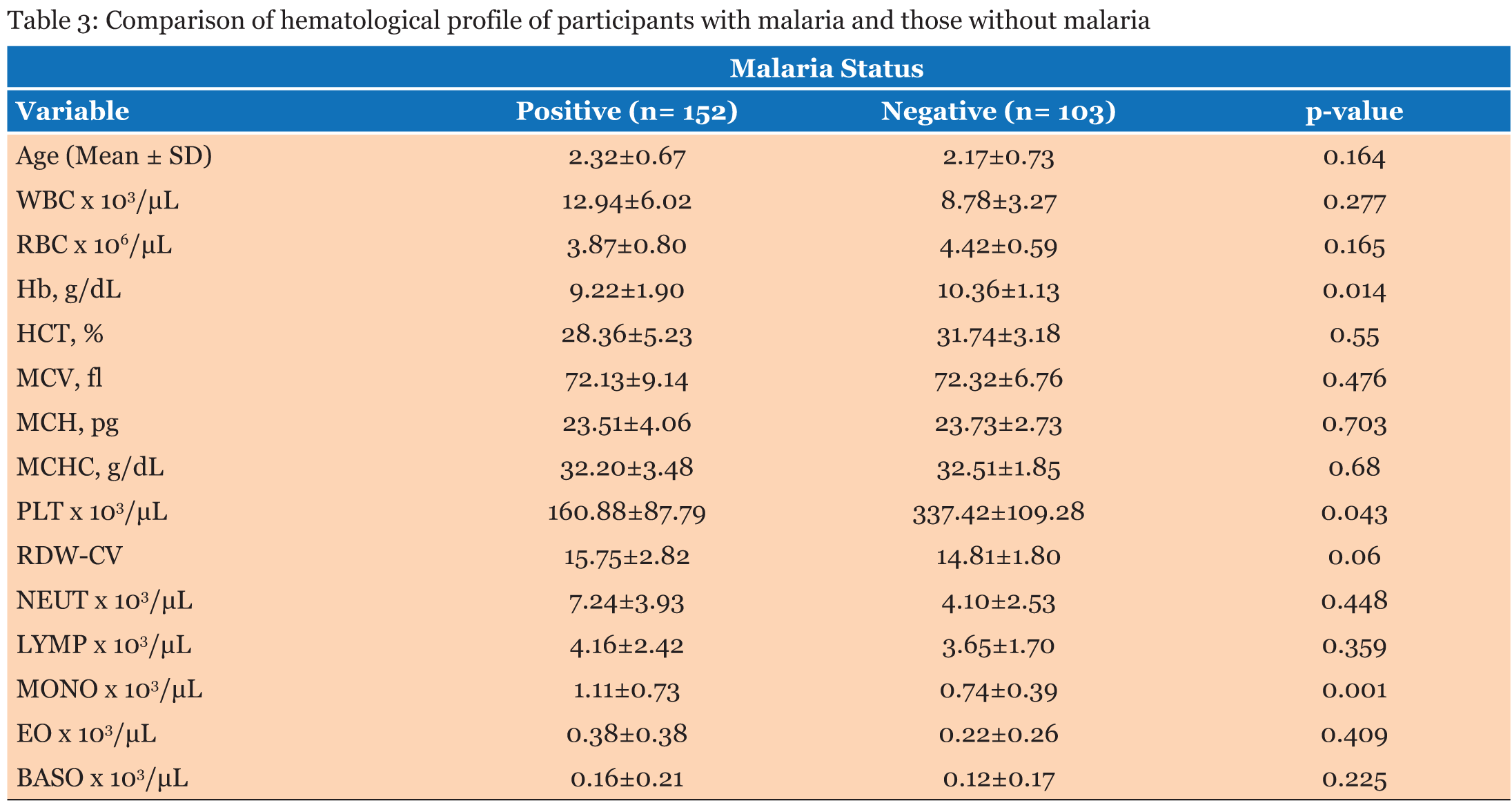
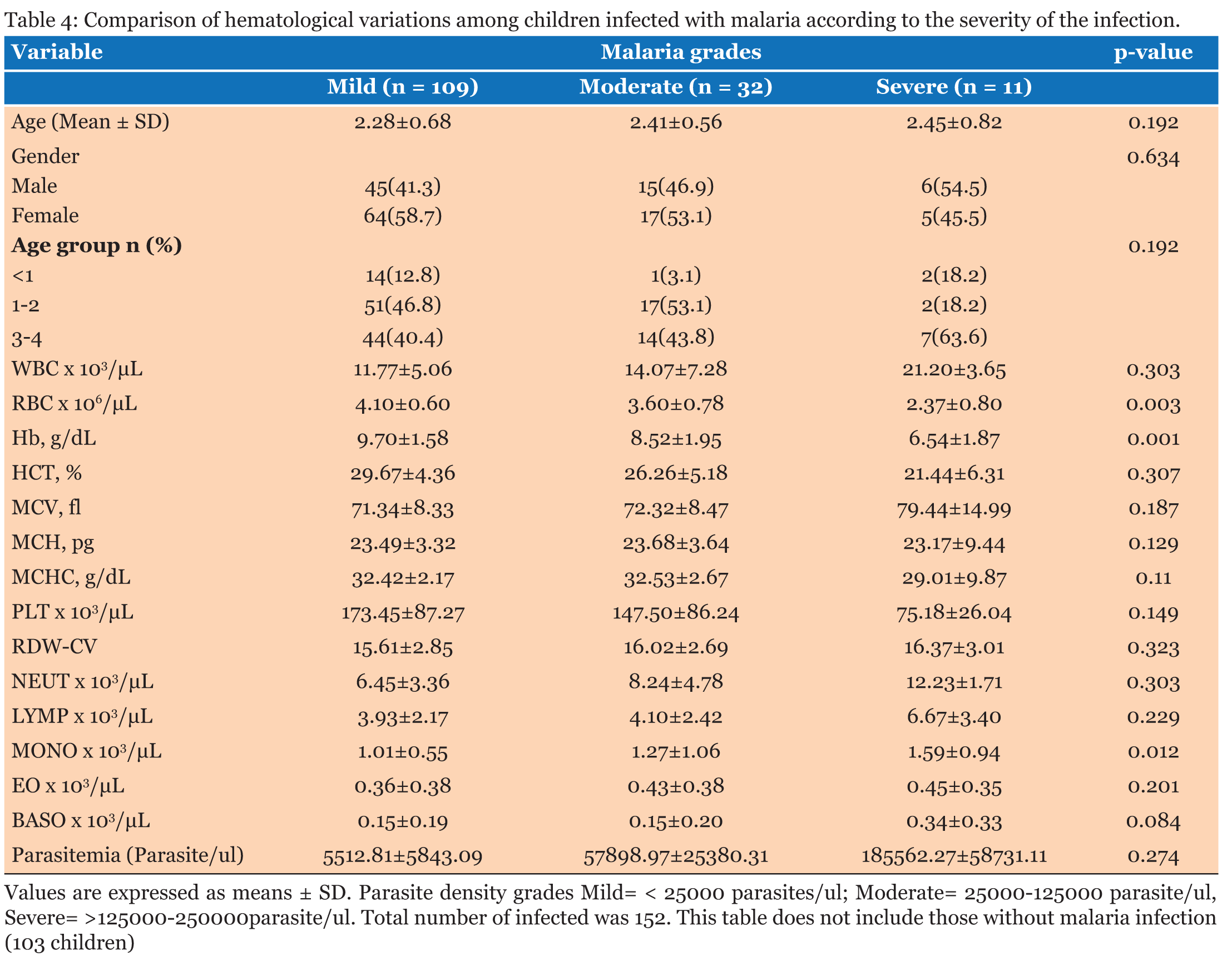
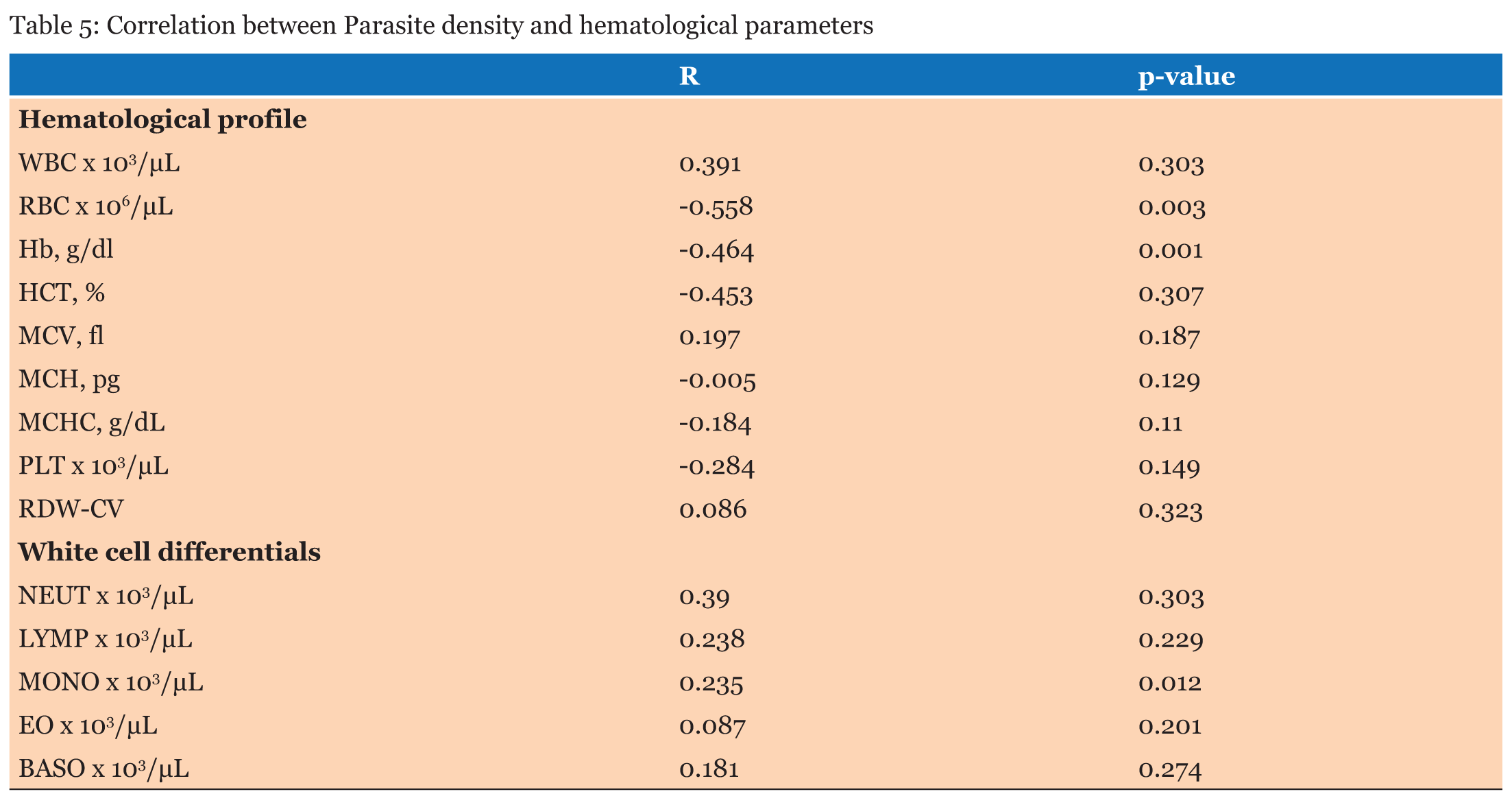
The study population involved 255 children aged below five years who reported to the hospital with fever and other symptoms leading to the primary diagnosis of malaria. A total of 123, (48.2%) were males and 132, (51.8 %) were females (Table 1). The average age of all the patients was 2.25 years and the median age was 1-2 years. The average age of the males was 2.24 years and that of the female was 2.28 years. When the hematological profile of all participants was stratified into gender there were no significant differences in all the parameters (Table 2). Participants with malaria were older than those without malaria, however, there was no significant difference in their ages. The hemoglobin concentration (Hb) concentration (P = 0.014) and the platelet count (P = 0.043) were both significantly lower in the cases than the controls while the monocyte count was also significantly higher among participants with malaria infection than those without malaria (P = 0.001). The rest of the parameters did not show significant difference between those with malaria and those without malaria (p>0.05) (Table 3). The RBC count and the Hb level decreased significantly with severity of malaria; P = 0.003 and P = 0.001 respectively, and the monocyte count increased significantly with severity of disease (P = 0.012). No significant differences were observed in the rest of the Full Blood Count parameters (P>0.05) (Table 4). The study revealed negative correlation between parasite density and hemoglobin concentration and positive correlation between monocyte count and parasite density. Though these correlations were significant, they were weak (Table 5).The hematological profile did not show significant variation with age among those infected with malaria parasites (P>0.05) (Table 6). | ||||||
| ||||||
| ||||||
| ||||||
| ||||||
| ||||||
| ||||||
|
DISCUSSION
| ||||||
|
This study examined the hematological profile of children under five years who were clinically diagnosed with malaria in the Volta Regional Hospital and the Ho Municipal Hospital. Children under five year’s are vulnerable with low immunity against the plasmodium parasite and so when they are infected with malaria, it could lead to anemia with the consequent morbidity and mortality. There were no significant differences in the full blood count parameters between the males and females. Furthermore, there was no significant difference between the various age groups with regards to the hematological profiles. White blood cells play vital role in the defense against malaria hence this study demonstrated overall increase in white blood cell numbers among participants with malaria as against those without malaria though the increase was not statistically significant. This is consistent with the findings of Maina and colleagues which found significant increase in WBC count in children with malaria compared to the controls in a cross-sectional study among children in Kisumu, western Kenya [15]. White blood cell changes in malaria are variable depending on factors like; parasitemia, host immunity state and the existence of co-infections [16]. Part of the body’s immune response to infections involves stimulation of effector cells which may be neutrophils, macrophages or Natural killer (NK) cells [17]. Consequently, reticuloendothelial hyperplasia, is one of the most significant early pathological changes in malaria parasitemia [16]. Monocytosis is one of the most significant observations reported from a previous study on hematological changes among children infected with malaria [18]. This is in consonant with the findings in the present study, where a significant increase in monocyte count was observed in parasitemic patients compared to the non-parasitemic patients. The mean neutrophil count between the parasitemic and non-parasitemic children in this study was not significantly different. These findings are similar to those from previous studies in India [19] and Singapore [7] respectively where they reported no significant increase in neutrophil count between those with malaria and those without malaria. The pathophysiology of neutropenia in malaria has been suggested to involve amplified margination and sequestration of neutrophils as a result of the augmented expression of cell adhesion molecules (ICAM-1 and VCAM-1) that occurs in malaria [20]. Available literature has indicated that lymphocyte count remains unchanged during an acute malaria infection [18]. This is in line with this study in which no significant difference in lymphocyte count in patients with malaria parasitemia and those without parasitemia is reported. The variations seen in the total lymphocyte count in malaria parasitemic patients may be due to either an elevation or a reduction in the numbers of differential white blood cell (WBC) lines. Also, the eosinophils and basophils counts were not significantly different between those with malaria and those without malaria in this study. In this study, the mean platelet counts in patients with malaria was normal, however, it was significantly lower than that of those without malaria. These observations may imply the likelihood of thrombocytopenia being an indicator of Plasmodium infection. The relationship between platelet count and plasmodium infection has been reported by a previous study [15]. Thrombocytopenia may occur probably as a result of destruction and removal of platelets by spleen in addition to platelet depletion by disseminated intravascular coagulopathy [21]. Elevation in the number of megakaryocytes in the bone marrow causes reduction in the production of platelets which may cause thrombocytopenia in malaria [22]. The destruction of platelets by the immune system has been hypothesized as a causative agent of thrombocytopenia seen in malaria infection [21]. Malaria-infected patients have raised levels of immunoglobulin G (IgG) in the blood which binds to platelet-bound malaria antigens leading to increased destruction of thrombocytes [23]. One study has shown that aggregated platelets are falsely counted as single platelet by the analyzers thus causing pseudo-thrombocytopenia [22]. The sustainability of plasmodium parasites is only possible when the parasites infect red blood cells of their human host. Therefore, changes in RBC indices are common in malaria infection [14]. Anemia is one of the commonest complications in malaria infection especially in pregnant women and children of school going age living in endemic areas [10]. The pathophysiology of anemia caused by plasmodium parasites is yet to be fully understood. However, it is hypothesized that the parasites target the red blood cells resulting in RBCs destruction. Furthermore, accelerated removal of both parasitized and non-parasitized RBCs, bone marrow dysfunction and the level of parasitemia all contribute to anemia in infected individuals [22]. In this study, there were significant decreases in the level Hb and erythrocyte count. This is in consonant with one study which stated that anemia is usually associated with Plasmodium infection especially in children and it is suggested to be caused by the destruction of infected RBCs and a decrease in hematopoiesis [24]. Mean cell volume, MCH, MCHC and RDW levels in patients infected with malaria were not significantly different from those without malaria. This could probably have been because uncomplicated malaria is associated with minor biochemical changes, such as lower production of cytokines, decreased endothelial cell activation, minor alterations in the coagulation profile, reduced sequestration, and fewer hemolysis as opposed to complicated malaria [14]. | ||||||
|
CONCLUSION
| ||||||
|
This study has demonstrated that malaria parasites affect the hematopoiesis in children under five years living in malaria-endemic areas. The greatest changes observed were thrombocytopenia, anemia and monocytosis. These changes may be useful and helpful in the diagnosis of malaria especially in cases of low level of parasitemia in malaria endemic areas. Therefore, analysis of hematological profile of patients suspected of having malaria will significantly improve malaria diagnosis when used in combination with other clinical and microscopic examination. These findings suggest that special attention should be given to these children as anemia can lead to cognitive impairment and developmental abnormalities. | ||||||
|
REFERENCES
| ||||||
| ||||||
|
[HTML Abstract]
[PDF Full Text]
| |||
|
Acknowledgements
The researchers are grateful to the Department of Medical Laboratory Science, University of Health and Allied Science and the management of the Volta Regional Hospital, Ho, Ghana for granting them permission to carry out the project in the facility. | |||
|
Author Contributions
Ahmed Tijani Bawah – Substantial contributions to conception and design, Acquisition of data, Analysis and interpretation of data, Drafting the article, Revising it critically for important intellectual content, Final approval of the version to be published Kofi Theodore Nyakpo – Substantial contributions to conception and design, Acquisition of data, Analysis and interpretation of data, Drafting the article, Revising it critically for important intellectual content, Final approval of the version to be published Francis Abeku Ussher – Substantial contributions to conception and design, Acquisition of data, Analysis and interpretation of data, Drafting the article, Revising it critically for important intellectual content, Final approval of the version to be published Huseini Alidu – Substantial contributions to conception and design, Acquisition of data, Analysis and interpretation of data, Drafting the article, Revising it critically for important intellectual content, Final approval of the version to be published Jerry Jones Dzogbo – Substantial contributions to conception and design, Acquisition of data, Analysis and interpretation of data, Drafting the article, Revising it critically for important intellectual content, Final approval of the version to be published Sampson Agbemenya – Substantial contributions to conception and design, Acquisition of data, Analysis and interpretation of data, Drafting the article, Revising it critically for important intellectual content, Final approval of the version to be published David Annor Kwasie – Substantial contributions to conception and design, Acquisition of data, Analysis and interpretation of data, Drafting the article, Revising it critically for important intellectual content, Final approval of the version to be published Mohammed Mustapha Seini – Substantial contributions to conception and design, Acquisition of data, Analysis and interpretation of data, Drafting the article, Revising it critically for important intellectual content, Final approval of the version to be published | |||
|
Guarantor of Submission
The corresponding author is the guarantor of submission. | |||
|
Source of Support
None | |||
|
Consent Statement
Written informed consent was obtained from the patient for publication of this study. | |||
|
Conflict of Interest
Author declares no conflict of interest. | |||
|
Copyright
© 2018 Ahmed Tijani Bawah et al. This article is distributed under the terms of Creative Commons Attribution License which permits unrestricted use, distribution and reproduction in any medium provided the original author(s) and original publisher are properly credited. Please see the copyright policy on the journal website for more information. | |||
About the Authors | |||
| |||
| |||
| |||
| |||
| |||
| |||
|
|