|
Research Article
Determinants and transfusion practices in pediatric critical care unit: A prospective study in thrombocytopenic children
1 MD, MBA, Head, Department of Transfusion Medicine and Immunohematology, Sri Balaji Action Medical Institute, A-4, Paschim Vihar, New Delhi, India
2 MD, Head, Pediatric Intensive Care Unit, Sri Balaji Action Medical Institute, A-4, Paschim Vihar, New Delhi, India
3 DNB, Department of Transfusion Medicine and Immunohematology, Sri Balaji Action Medical Institute, A-4, Paschim Vihar, New Delhi, India
Address correspondence to:
Sadhana Mangwana
Department of Transfusion Medicine and Immunohematology, Sri Balaji Action Medical Institute, A-4, Paschim Vihar, New Delhi 110063,
India
Message to Corresponding Author
Article ID: 100012P05SM2021
Access full text article on other devices

Access PDF of article on other devices

How to cite this article
Mangwana S, Sharma PK, Lavanya B. Determinants and transfusion practices in pediatric critical care unit: A prospective study in thrombocytopenic children. Edorium J Pediatr 2021;5:100012P05SM2021.ABSTRACT
Aims: To evaluate incidence of thrombocytopenia, transfusion requirement, outcome, and prognostic value of platelet count. Prevalence of thrombocytopenia varies in various Intensive Care Units, ranging from 13% to 58% depending on disease profile of patients. Platelet count is considered a predictor of outcome. Restrictive transfusion policies save transfusion recipients from inherent risks of transfusion adverse reactions.
Methods: Prospective study was conducted over 15 months. Total 450 children of age >1 month and < 18 years in Pediatric Intensive Care Unit and High Dependency Unit were included. Patients’ complaints, complications, laboratory data, length of stay, severity score, transfusion requirement, and outcome were recorded.
Results: Patients’ age ranged from 4 months to 15 years with male preponderance. Incidence of thrombocytopenia was 24%. Fever, shock, and bleeding manifestations were found in 70.44%, 5.77%, and 9.11% cases, respectively; more in thrombocytopenia patients. Circulatory failure and shock were found in 17.59% thrombocytopenic patients vs. 2.04% cases in non-thrombocytopenic patients. Single- and multi-organ failure were more in thrombocytopenia patients (34.25% and 12.96%, respectively). 99.89% patients were discharged. Mortality rate of 3.7% in thrombocytopenia against 0.29% in non-thrombocytopenia patients support that thrombocytopenia is a good prognostic indicator for outcome. 4.44% patients required transfusion. 2.77% thrombocytopenia patients required multiple component transfusion and 0.92% cases only platelet transfusion; reinforcing conservative transfusion policies.
Conclusion: Thrombocytopenia is identified as prognostic indicator and major factor for bleeding and mortality in critically ill patients. It is concluded that restrictive strategy for platelet transfusions is safe and patients should be treated for underlying causes and monitored closely. There is need to define pediatric platelet transfusion thresholds for various clinical settings.
Keywords: Organ failure, Outcome, Platelet, Prism score, Thrombocytopenia, Thrombocytopenia associated multi organ failure (TAMOF), Transfusion, Viral markers
INTRODUCTION
Thrombocytopenia is a condition resulting from underproduction, destruction, or over consumption of platelets [1]. Prevalence of thrombocytopenia varies in various ICU, ranging from 13% to 58% depending on the disease profile of patients [2], [3]. Although highest rates are seen in septic and trauma patients; bleeding, transfusions, certain drugs, intravascular catheters, shock, acute respiratory distress syndrome (ARDS), and disseminated intravascular coagulation (DIC) are other risk factors associated with thrombocytopenia [4]. The platelet count is now considered to be a predictor of outcome [4].
An inverse correlation of the platelet count is observed with the risks for a prolonged ICU stay and mortality; mortality rate of 31–46% vs. 16–20% (in thrombocytopenic patients vs. non-thrombocytopenic patients) [5],[6],[7]. When ICU patients are presented with thrombocytopenia at admission or subsequently develop, more severe disease or more organ failures can be predicted [2]. Platelet transfusions show varied response to transfusion. American Association of Blood Banks (AABB) has issued clinical practice guidelines for prophylactic platelet transfusions in adults to prevent spontaneous bleeding, based on platelet counts; according to which, platelet transfusion is recommended in hospitalized adult patient with 10×109 cells/L or less while 20×109 cells/L for elective central venous catheter insertion, 50×109 cells/L for elective lumbar puncture and major neurosurgeries [8]. Thresholds for prophylactic platelet transfusion in pediatrics population are not well established [9]. Pending additional pediatrics specific studies and guidelines, prophylactic platelet transfusion guidelines in adults issued by AABB in 2014 are generally considered.
MATERIALS AND METHODS
A prospective, observational study was conducted between October 2018 and December 2019 in which 450 children of age >1 month and < 18 years in level III Pediatric Intensive Care Unit (PICU) and level II High Dependency Unit (HDU) of a tertiary care hospital from capital city were enrolled who were admitted for minimum of 24 hours and more. Post-operative patients and patients who were transferred from wards to ICU were excluded from the study. Patients’ presenting complaints and complications, laboratory data were recorded at the time of admission and during hospital stay along with demography, primary diagnosis, severity score (PRISM III score), length of stay in ICU and HDU, and outcome in terms of discharged or expired. All patients who were discharged from ICU, transferred out to ward or patients who left against medical advice (LAMA) were considered as discharged patients.
Thrombocytopenia is defined as platelet count below 150×109/L. The severity of thrombocytopenia was classified as mild, moderate, severe, and very severe depending on platelet count as 100 to 149×109/L, 50 to 99×109/L, <50×109/L, and <20×109/L, respectively [4].
Data were analyzed by appropriate statistical tools.
The Study was approved by Institutional Ethics Committee vide reference no. SBAMI-EC/2018/57.
RESULTS
Patients’ age in this study varied from 4 months to 15 years (Mean age as 5.79 ± 3.48 years) with male preponderance (61.1%, n=275). Incidence of thrombocytopenia was 24% (n=108). 20.22% (n=91) patients presented with thrombocytopenia at admission and 3.78% patients (n=17) developed thrombocytopenia during hospital stay; henceforth named as Group 1 and Group 2 respectively. When these cases were analyzed into 3 age groups as 0–5 years, 5–10 years, and 10–15 years, there was no specific gender distribution except significant male preponderance in very severe category of thrombocytopenia. Incidence of mild, moderate, severe, and very severe thrombocytopenia among Group 1 patients was 41.76% (n=38), 27.46% (n=25), 24.18% (n=22), and 6.6% (n=6) respectively, while its incidence in Group 2 patients was 58.8% (n=10), 23.5% (n=4), 11.8% (n=2), and 5.9% (n=1) respectively.
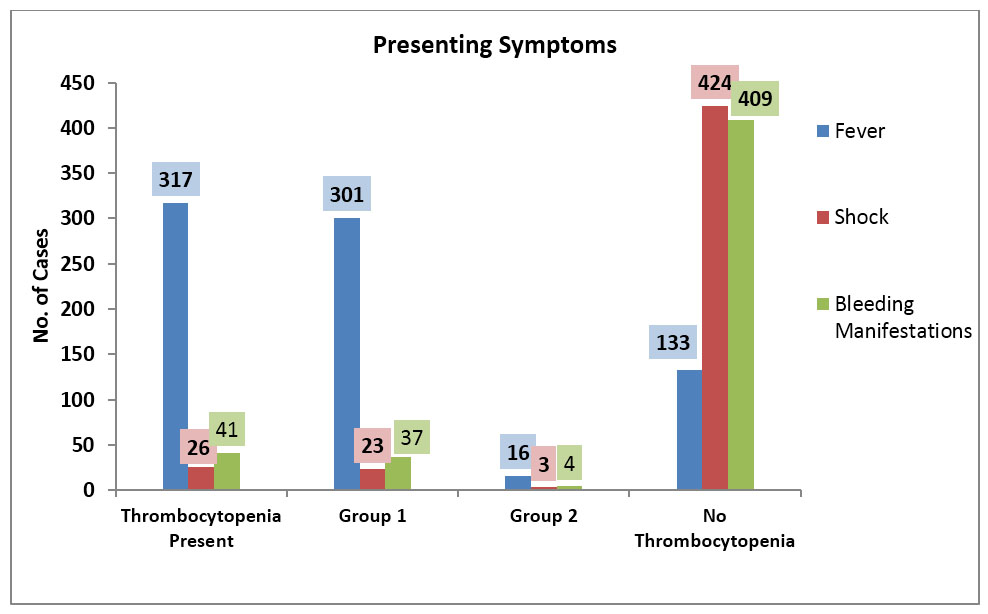
Fever, shock, and bleeding manifestations, as presenting symptoms, in all admitted children in PICU and HDU were found in 70.44% (n=317), 5.77% (n=26), and 9.11% (n=41) cases respectively, more in thrombocytopenia patients than in non-thrombocytopenia patients (Figure 1). Fever was found maximum in very severe and severe thrombocytopenia cases of Group 1; 100% in both categories (n=6 and n=22 respectively) followed by moderate (96%; n=24), mild (81.58%; n=31) and non-thrombocytopenia patients (60.72%; n=218) (p value <0.01). Among Group 2 patients, fever was present in 100% cases in mild, moderate, and severe categories while it was not a presenting symptom in very severe category (p value <0.01).
Shock was present in seven patients of non-thrombocytopenia category, 16 in Group 1, and three in Group 2 category of thrombocytopenia patients. Highest number of cases (50%; n= 3) were found in very severe category, followed by severe (36.36%; n=8), moderate (12.0% 3), mild (5.26%; n=2), and non-thrombocytopenic category (1.95%; n=7) (p value <0.01). In Group 2, three patients who developed shock belonged to mild (10%), moderate (25%), and very severe category (100%) (p value <0.008).
Bleeding manifestations were presented maximum in very severe category (50%) followed by severe (31.82%), moderate (20%), and mild (5.26%) thrombocytopenia patients of Group 1 respectively while in non-thrombocytopenia patients, bleeding manifestations were present in 5.57% cases (p value <0.01). In Group 2 patients, only four patients showed bleeding manifestations, of which 2 cases each belonged to mild and severe categories. Comparison between Group 1 and Group 2 with bleeding manifestation showed statistically significant difference (p value 0.04).
Coagulopathy was found in 27.78% of thrombocytopenia patients with raised activated partial thromboplastin time (APTT) which increased with severity of thrombocytopenia (p value 0.04).
There was no definitive correlation of liver function tests (LFT) with degree of severity of thrombocytopenia except deranged parameters in viral hepatitis cases.
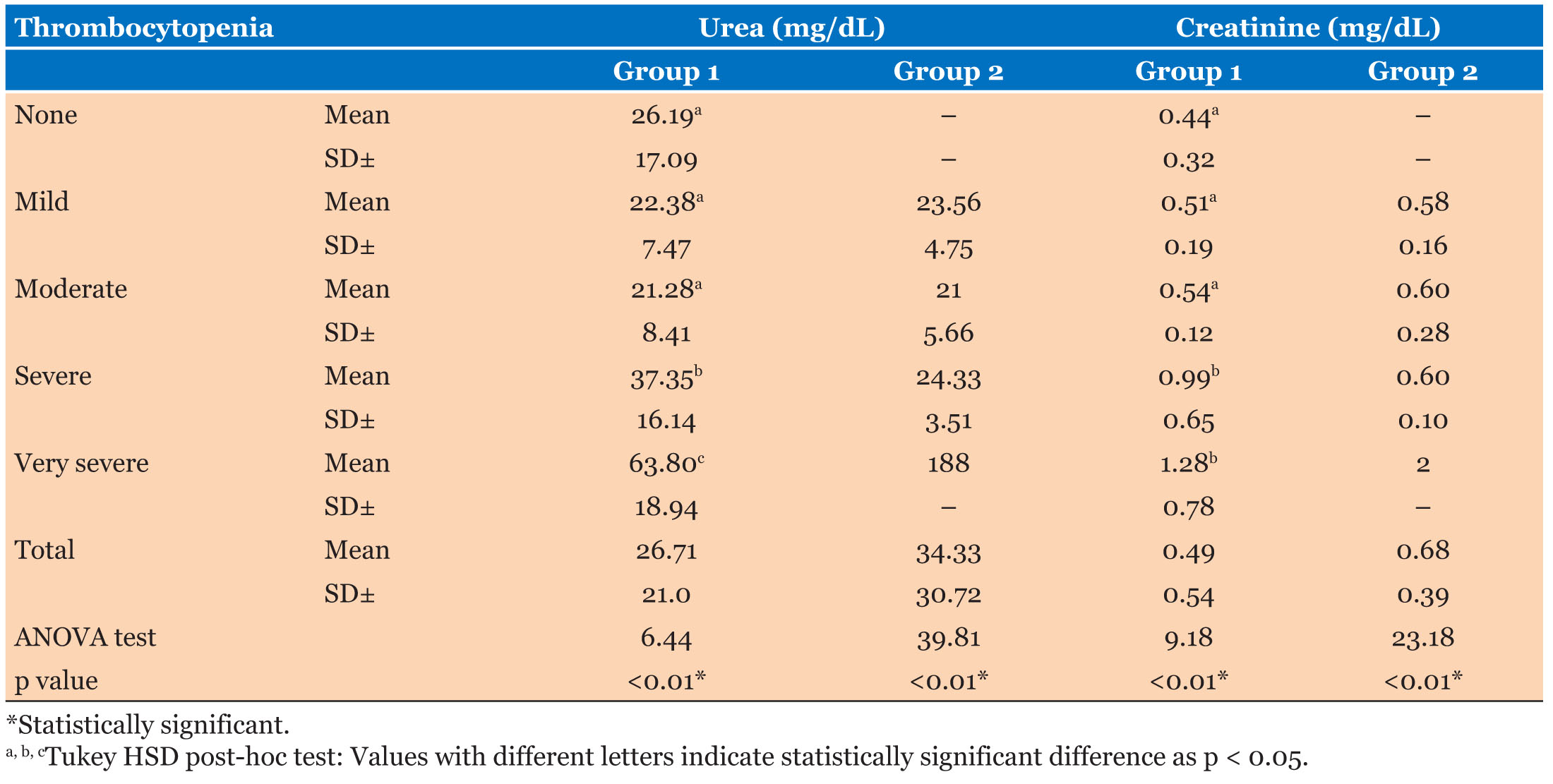
Kidneys were found to be affected in 61.11% patients of thrombocytopenia. Blood urea and serum creatinine levels were markedly increased in very severe and severe categories of Group 1 followed by moderate and mild categories (p value <0.01) indicating impact on renal function with increasing severity of thrombocytopenia. In Group 2, mean urea and creatinine levels were found maximum in very severe category followed by severe, mild, and moderate categories (p value < 0.01) (Table 1).
In this study, commonest diagnosis among the thrombocytopenia patients, in order of frequency, was dengue (56.48%; n=61) followed by sepsis (10.18%; n=11), encephalopathy (7.41%; n=8), enteric fever (5.56%; n=6), acute gastroenteritis (3.7%; n=4), meningitis, hepatitis, Road Traffic Accidents (RTA) (2.78% each; n=3 each), Idiopathic Thrombocytopenic Purpura (ITP), pneumonia (1.85% each; n=2 each), swine flu, lower respiratory tract infection and renal disease (0.92% each; n=1 each).
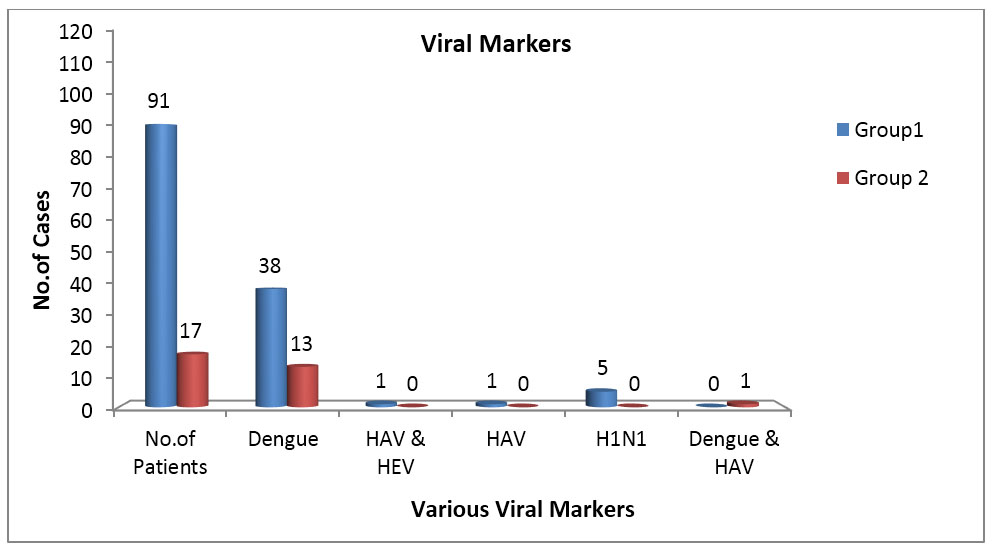
Tests conducted for viral markers after admission showed presence of dengue in 47.22% with 41.76% (n=38) of Group 1 thrombocytopenia patients and 76.47% (n=13) of Group 2 thrombocytopenia category. In Group 1, one case each showed positivity for both hepatitis A virus (HAV) and hepatitis E virus (HEV) (4%) and H1N1 influenza positivity (4%) in moderate category while HAV alone was positive in an isolated case of mild category (2.63%). In Group 2, one case showed positivity for both dengue and HAV (10%) in mild category. Association of viral markers increased with severity of thrombocytopenia in Group 1 from mild (21%) to moderate (60%) to severe category (72.7%) while in Group 2, from mild (80%) to moderate and severe categories (100% each), especially dengue being the most important and significant cause leading to thrombocytopenia in both the groups (Figure 2).
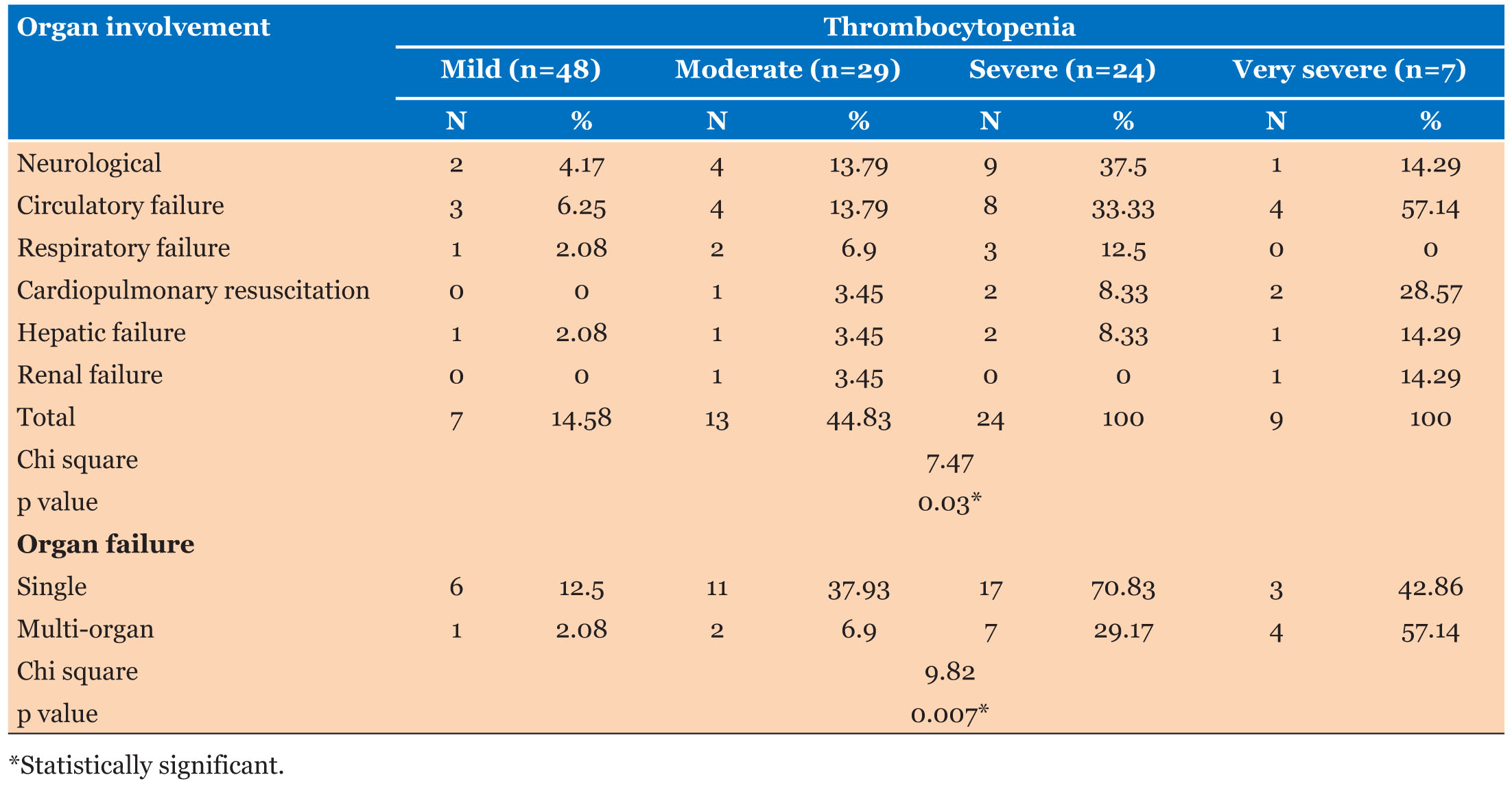
In all thrombocytopenic patients (n=108), Organ involvement was observed in ascending order, from mild (14.58%) to moderate (44.83%) to severe (100%) and very severe (100%) categories (Table 2). Single- and multi-organ failures in thrombocytopenia patients were found as 34.25% and 12.96%, respectively. Circulatory failure was seen in 17.59% cases (n=19) of which five cases required cardiopulmonary resuscitation (CPR); one in moderate category and two cases each in severe and very severe category. Neurological involvement was seen in 14.81% cases (n=16) while hepatic failure was seen in 4.63% cases and renal failure in 1.85% (p value <0.03). Single organ failure was found maximum in severe category (70.83%) followed by very severe (42.86%), moderate category (37.93%). Multi-organ failure was highest in very severe patients (nearly 25%); out of these, 3.70% patients had more than 10 days stay.
PRISM III score, indicating outcome of the patient, was found maximum among moderate category of Group 1 (5.96±3.69) followed by severe (5.91±2.84), very severe (5.50±2.35), mild category (2.61±1.48) thrombocytopenia cases and in non-thrombocytopenia patients (1.12±2.24) (p value <0.05). Among Group 2 thrombocytopenia cases, PRISM III score was found to be highest (17±0) in very severe category, followed by mild (2.1±1.91), moderate (1.5±1), and severe (1±1.41) category patients, respectively. Comparison between the two groups showed statistically significant difference in all the categories (p value < 0.02) indicating PRISM score had significant correlation with the severity of thrombocytopenia.
Majority of patients (99.89%; n=445) were discharged and only 1.11% (n=5) could not be saved, of which four cases were male and one female patient. Mortality was very low in non-thrombocytopenia patients (0.29%, n=1), than in thrombocytopenic cases (3.7%, n=4), which was highest in very severe cases followed by severe category (57.14%) followed by severe (29.17%), moderate (6.9%), and mild (2.08%) thrombocytopenia patients (p value <0.007). Thrombocytopenia-associated multiple organ failure (TAMOF) was found in 12.96% (n=14 patients) with highest number of cases showing cardiac involvement followed by neurological and respiratory organs, related with sepsis, leading to failure.
Length of stay was more in thrombocytopenic patients as compared to non-thrombocytopenia patients. More than 34% patients with thrombocytopenia stayed for six days and above as compared to non-thrombocytopenia and moderate categories (p value < 0.001). These four thrombocytopenic patients had developed TAMOF, with majority (n=3) belonged to age group 0–5 years and only one patient was in 10–15 year age group who was, although having moderate thrombocytopenia, a known case of acyanotic congenital heart disease, acute myocarditis, and shock which led to unfavorable outcome.
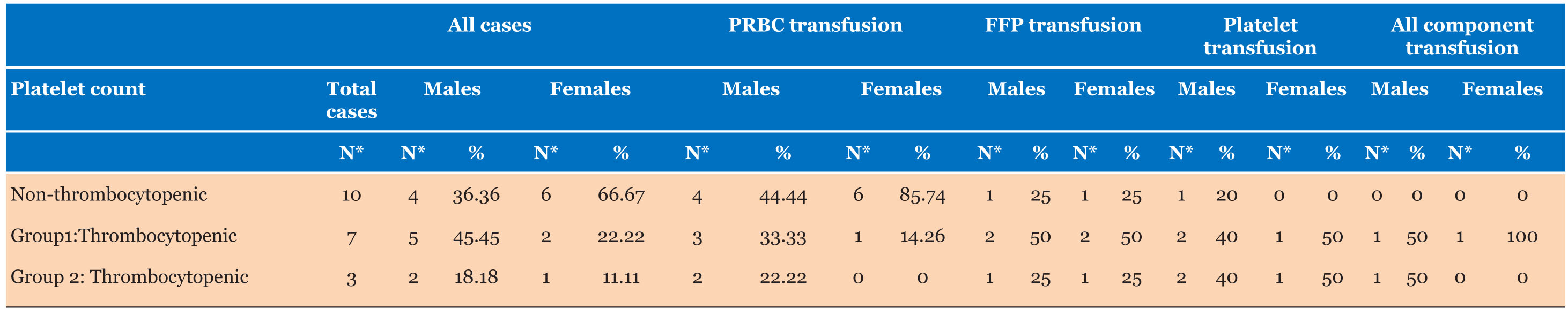
Only 4.44% of total patients (n=20) required transfusion of which 50% children (n=10) were in non-thrombocytopenia category who received only red cell transfusions (40% males and 60% females) making 2.78% of non-thrombocytopenia category while 10.98% patients of thrombocytopenia category (n=10) required transfusion (p value <0.01). Seven patients from Group 1 (71% males and 29% females) and three patients of Group 2 (67% males and 33%females) required transfusion. 2.77% (n=3) thrombocytopenia patients required multiple component transfusion and only one case (0.92%) required platelet transfusion. All patients of non-thrombocytopenia category requiring only red cell transfusion were diagnosed as anemia, pneumonia, trauma, and osteomyelitis. In Group 1 thrombocytopenia category, four patients required red cell transfusion, four patients required fresh frozen plasma (FFP), and three patients required platelet transfusion. Two patients requiring all components had multi-organ failure; one consequent to septic shock and other with diagnosis of dengue fever. Of three patients in Group 2 thrombocytopenia category, two patients required red cell transfusion, two patients required FFP transfusion, and all three patients required platelet transfusion (Table 3). Only one patient required all component transfusion that was diagnosed as severe sepsis, shock, disseminated intravascular coagulation (DIC) with multi-organ failure.
Transfused patients had blood group frequencies, in descending order, as ‘O’ positive, ‘B’ positive (n=7 each), ‘A’ positive (n=3), ‘AB’ positive, ‘A’ negative, ‘B’ negative (n=1 each). Extended RH phenotypes in transfused patients showed frequencies of various antigens as ‘e’—100%, ‘C’ and ‘c’—68.4% each with ‘E’—15.8%. Kell phenotype was negative in all transfused patients. According to Weiner and Fischer-Race classification, maximum patients showed phenotypic frequencies as R1r/CcDee (36.8%) followed by R1R1/CCDee (31.6%), R2r/ccDEe (21.1%), and rr/ccdee (11%).
DISCUSSION
Incidence of thrombocytopenia in this study is found to be 24%; comparable with other studies (25% and 17.3% respectively) [3],[10], but lower than that of Al-Marzoki Jasim et al. and Yilmaz et al. (44.61% and 59.57% respectively) [11],[2]; having male preponderance, similar to other studies [3],[12].
Fever was the commonest presenting symptom (70.44%), similar to other studies which also found fever as the major complaint (100% cases in both) [13], [14]. Circulatory failure and shock were found in total 17.59% thrombocytopenic patients and only 2.04% non-thrombocytopenia patients developed shock. Cardiopulmonary resuscitation requirement in shock cases was very less (4.62%) as compared to Agarwal et al. (31.4 %) [3]. Shock was present in 57% cases of very severe category, finding similar toVenkata et al. who found that 47.6% of severe sepsis developed thrombocytopenia of which 93.1% had septic shock, a finding similar to Group 2 thrombocytopenia patients in this study (100%) [15]. Bleeding manifestations (9.11%) were similar to various studies in adult patients (9.43%, 15%, 15.11%, 19%, 4%, and 19.45% respectively) [13],[16],[17],[18],[19],[20]. As per WHO classification of bleeding into 4 grades [8], 61.9% thrombocytopenia patients in this study had bleeding grade 1 and 38.1% thrombocytopenia patients had bleeding patients grade 2. Makroo et al. observed that hemorrhagic manifestations occurred more in adult patients with severe thrombocytopenia and frequent when platelet counts were less than 20,000/mm3 [17]; similar finding of bleeding manifestations in 98.46%, 54%, and 17.97% in severe, moderate, and mild thrombocytopenia patients respectively were noted by Tejas et al. while Nair et al. observed bleeding manifestations in 77.14%, 3.89%, and 0.56% cases in very severe, severe, mild categories respectively [14],[19]. Strauss et al. and Venkata et al. found more bleeding incidence (33%, 14.4% ) in cases with thrombocytopenia as compared to non-thrombocytopenia cases (9%, 3.7%) respectively [21],[15]; similar to observations in this study.
Incidence of coagulopathy in this study in thrombocytopenia patients is lower (27.78%) than other studies in literature (43% and 73% respectively) [3],[2], indicating early detection and correction of deranged coagulation profile in our high level critical care.
Single- and multi-organ failures leading to TAMOF showed highest cardiac involvement followed by neurological and respiratory involvement, related more with sepsis. Incidence of TAMOF in this study is lower (12.96%) than that of Chakradhar et al. (>44%) who observed higher incidence of acute lung injury (ALI) or acute respiratory distress syndrome (ARDS) with increasing severity of thrombocytopenia [15]; indicating high levels of critical care in our set up.
Febrile thrombocytopenia was the commonest cause in this study which led to the diagnosis of dengue, similar to Gondhali et al. and Suneetha et al. [16],[19]. Many patients were investigated on outpatient’s basis and diagnosed as dengue before admission to our facility leading to difference in numbers of final diagnosis and viral markers. Sepsis was the second commonest diagnosis (10.18%) in this study which is lesser than many studies [12],[16],[22]. It has long been recognized that thrombocytopenia may be an early warning sign of sepsis. Although mechanism of thrombocytopenia in sepsis not completely clear, but it has been proposed that hemophagocytosis may play an important role in patients with sepsis [2],[22],[23].
Association of viral markers, especially dengue being the most important and commonest cause associated with thrombocytopenia, is in accordance with findings of many authors [24],[25],[26]. Presence of various viral markers, i.e., dengue, dual positivity of dengue with HAV, and HAV with HEV indicates that virus can trigger decrease in platelet production by the infection of megakaryocytes which can lead to apoptosis [27].
Length of stay of >10 days was more in thrombocytopenia cases than in non-thrombocytopenia cases (3.7% vs. 2.63%); in accordance with observation by many authors [2],[4],[20]. There was a negative correlation between platelet count and duration of hospital stay.
PRISM score had significant correlation with the severity of thrombocytopenia. Nearly 14% thrombocytopenia patients had high PRISM score III (more than 8); much lower than Agarwal et al. who observed PRISM score of >8 in 82.8% in thrombocytopenia patients and 28% in non-thrombocytopenia patients. This difference could be due to more number of cases of dengue and HDU admissions in our study while sepsis cases were more in the study of Agarwal et al. [3].
Outcome was very favorable in our study. Mortality rate of 3.7% and 0.29% in thrombocytopenia and non-thrombocytopenia patients respectively support that thrombocytopenia is a good prognostic indicator for outcome. This finding is in accordance with earlier observation [2] which found increasing severity of thrombocytopenia was associated with increased mortality, mainly in septic patients due to multiple organ dysfunction (MODS) [12], [21]. Only one non-thrombocytopenic patient in our study didn’t survive who was admitted with severe trauma while other four patients, who could not be saved, had developed TAMOF, though majority of patients with TAMOF (71%, n=10) were discharged indicating high level of pediatric critical care in our setup.
In this study, total transfusion requirements were much lesser (4.44%) as compared to other studies where transfusion requirement in thrombocytopenia patients were 54% and 48.31% respectively [3],[12] reinforcing conservative transfusion policies. American Association of Blood Banks (AABB) and Society for Critical Care Medicine (SCCM) 2016 Surviving Sepsis Guideline recommends prophylactic platelet transfusion below a threshold of 10×109/L [8],[28] but there is no guideline for pediatric population. Hence, adult guidelines were followed in our pediatric critical care settings.
CONCLUSION
In this prospective, large-sized population study in level III PICU and level II Paediatric High Dependency Unit (PHDU) patients, thrombocytopenia has been identified as prognostic indicator and a major cause for bleeding and mortality. However, it does not predict and correlate with life-threatening bleeding. In this study, there were lesser number of hospital acquired infections (HAI) indicated by lower number of sepsis patients and lesser number of patients developing thrombocytopenia during hospital stay. Very less transfusion requirements reinforce that clinical practice guidelines (WHO/AABB) for platelet transfusions should be adhered and patients should be treated for underlying causes and monitored closely, which is evident in the form of single platelet transfusion given to a patient of dengue hemorrhagic fever (DHF) with polyserositis. Lesser number of transfusions saved patients from inherent risks of transfusion related adverse reactions. This study had certain limitations as well. It was a single center study and oncology or surgical pediatric patients were not included in this study. Thresholds for prophylactic platelet transfusion in pediatrics population are not well established. There are documented, well defined guidelines for adult platelet transfusions for various settings, this breakdown is remarkably absent for pediatric patients with the only division being neonatal vs. pediatric. The current prophylactic transfusion practice in the PICU is based on expert opinion and there is a lack of objective evidence to favor prophylactic vs. therapeutic platelet transfusion [9]. The optimum platelet count threshold for transfusion is significantly variable depending on the underlying etiology, clinical practice setting and geographic location which need further evaluation. Currently, there is no consensus for pediatric platelet transfusion threshold and much to be defined regarding optimal usage of platelet transfusion in pediatric patients, mainly critically ill children. We strongly recommend that platelet transfusions should be based on clinical practice guidelines and there is need to define pediatric platelet transfusion thresholds for various clinical settings for the benefit of patients and rationale use of precious human resources.
REFERENCES
1.
2.
3.
Agrawal S, Sachdev A, Gupta D, Chugh K. Platelet counts and outcome in the pediatric intensive care unit. Indian J Crit Care Med 2008;12(3):102–8. [CrossRef]
[Pubmed]

4.
Vanderschuren S, De Weerdt A, Malbrain M, et al. Thrombocytopenia and prognosis in intensive care. Crit Care Med 2000;28(6):1871–6.
[Pubmed]

5.
Drews RE. Critical issues in hematology: Anemia, thrombocytopenia, coagulopathy, and blood product transfusions in critically ill patients. Clin Chest Med 2003;24(4):607–22. [CrossRef]
[Pubmed]

6.
Greinacher A, Selleng K. Thrombocytopenia in the intensive care unit patient. Hematology Am Soc Hematol Educ Program 2010;2010:135–43. [CrossRef]
[Pubmed]

7.
Drews RE, Weinberger SE. Thrombocytopenic disorders in critically ill patients. Am J Respir Crit Care Med 2000;162(2 Pt 1):347–51. [CrossRef]
[Pubmed]

8.
Kaufman RM, Djulbegovic B, Gernsheimer T, et al. Platelet transfusion: A clinical practice guideline from the AABB. Ann Intern Med 2015;162(3):205–13. [CrossRef]
[Pubmed]

9.
Kahn S, Chegondi M, Nellis ME, Karam O. Overview of plasma and platelet transfusion in critically ill children. Front Pediatr 2020;8:601659. [CrossRef]
[Pubmed]

10.
Drews RE. Critical issues in hematology: Anemia, thrombocytopenia, coagulopathy, and blood product transfusions in critically ill patients. Clin Chest Med 2003;24(4):607–22. [CrossRef]
[Pubmed]

11.
12.
Kaur A, Sethi GK, Goyal RK, et al. Thrombocytopenia in paediatric ICU: Incidence, transfusion requirement and role as prognostic indicator. J Clin Diagn Res 2015;9(12):SC05–7. [CrossRef]
[Pubmed]

13.
Wayez A, Zafar L, Aijaz M, Afroz N. Study of platelet indices in dengue fever with thrombocytopenia and correlation of immature platelet fraction (IPF) with platelet recovery. Arch Hematol Case Rep Rev 2020;5(1):1–5. [CrossRef]

14.
15.
Venkata C, Kashyap R, Farmer JC, Afessa B. Thrombocytopenia in adult patients with sepsis: Incidence, risk factors, and its association with clinical outcome. J Intensive Care 2013;1(1):9. [CrossRef]
[Pubmed]

16.
17.
Makroo RN, Raina V, Kumar P, Kanth RK. Role of platelet transfusion in the management of dengue patients in a tertiary care hospital. Asian J Transfus Sci 2007;1(1):4–7. [CrossRef]
[Pubmed]

18.
19.
20.
21.
Strauss R, Wehler M, Mehler K, Kreitzer D, Koebnick C, Hahn EG. Thrombocytopenia in patients in the medical intensive care unit: Bleeding prevalence, transfusion requirement, and outcome. Crit Care Med 2002;30(8):176–71. [CrossRef]
[Pubmed]

22.
Levi M. Platelets in sepsis. Hematology 2005;10 Suppl 1:129–31. [CrossRef]
[Pubmed]

23.
Akca S, Haji-Michael P, de Mendonça A, Suter P, Levi M, Vincent JL. Time course of platelet counts in critically ill patients. Crit Care Med 2002;30(4):753–6. [CrossRef]
[Pubmed]

24.
Ingale SV, Upadhye AJ, Upadhye JJ. Correlation of serological markers and thrombocytopenia in early diagnosis of dengue infection. Int J Res Med Sci 2018;6(3):812–6. [CrossRef]

25.
Jyothi P, Metri BC. Correlation of serological markers and platelet count in the diagnosis of Dengue virus infection. Adv Biomed Res 2015;4:26. [CrossRef]
[Pubmed]

26.
Kulkarni RD, Patil SS, Ajantha GS, et al. Association of platelet count and serological markers of dengue infection—importance of NS1 antigen. Indian J Med Microbiol 2011;29(4):359–62. [CrossRef]
[Pubmed]

27.
Assinger A. Platelets and infection – An emerging role of platelets in viral infection. Front Immunol 2014;5:649. [CrossRef]
[Pubmed]

28.
Zarychanski R, Houston DS. Assessing thrombocytopenia in the intensive care unit: The past, present, and future. Hematology Am Soc Hematol Educ Program 2017;2017(1):660–6. [CrossRef]
[Pubmed]

SUPPORTING INFORMATION
Author Contributions
Sadhana Mangwana - Conception of the work, Design of the work, Analysis of data, Drafting the work, Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Pradeep Kumar Sharma - Conception of the work, Design of the work, Analysis of data, Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Bandaru Lavanya - Acquisition of data, Analysis of data, Drafting the work, Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Guaranter of SubmissionThe corresponding author is the guarantor of submission.
Source of SupportNone
Consent StatementWritten informed consent was obtained from the patient for publication of this article.
Data AvailabilityAll relevant data are within the paper and its Supporting Information files.
Conflict of InterestAuthors declare no conflict of interest.
Copyright© 2021 Sadhana Mangwana et al. This article is distributed under the terms of Creative Commons Attribution License which permits unrestricted use, distribution and reproduction in any medium provided the original author(s) and original publisher are properly credited. Please see the copyright policy on the journal website for more information.